Written and Illustrated by Mark Brundrett
CSIRO Forestry and Forest Products
Written and Illustrated by Mark Brundrett
CSIRO Forestry and Forest Products
 |
 |
 |
| This section introduces VAM associations produced by Glomalean fungi, illustrates structures produced by these fungi in roots and defines the terminology used to describe them. These associations are known as arbuscular mycorrhizas, VA mycorrhizas, endomycorrizas, or endotrophic mycorrhizas and are abbreviated as VAM here. There is disagreement about whether arbuscular mycorrhizas or vesicular-arbuscular mycorrhizas is the most appropriate name to use, because some fungi do not produce vesicles, arbuscules are not always present in roots and the role of arbuscules in nutrient exchange has not been confirmed (Smith 1995, Walker 1995). VAM associations involve fungi in the Zygomycete order Glomales and the roots of a wide diversity of plants. Features of spores are usually used to identify Glomalean fungi. Components of VAM associations are listed below. | |
|
|
 |
 |
 |
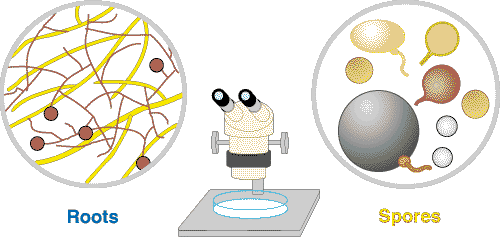
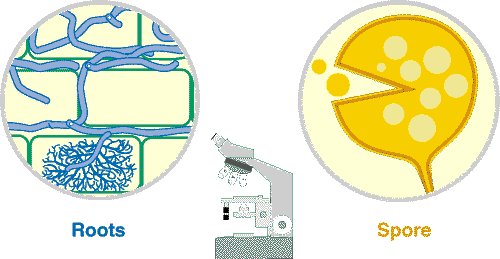
| These associations are introduced by 3 diagrams showing increasing magnification views. Information on the methods required to see mycorrhizal structures in Section 8. |

|
|

|
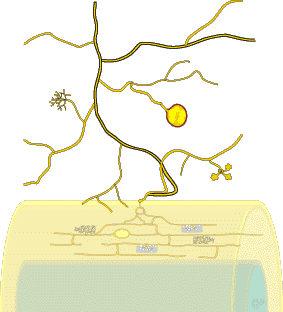
Mycorrhizal roots and the associated networks of hyphae are a major component of most soils, but structures produced by VAM fungi cannot normally be seen with the naked eye (they are greatly exaggerated in this diagram). |

|
Samples of roots or soil can be collected, processed and viewed with a microscope as described below to detect mycorrhizas. |
|
|

|
The medium level of magnification provided by a dissecting microscope allows hyphae and spores to be seen. These can be found by washing soil from roots very gently, or by using extraction procedures. |

|
Mycorrhizal structures within root are normally not visible, unless roots have been cleared in hot alkali to make them transparent and stained with a dye (Trypan blue or Chlorazal black E) that binds to fungal hyphae. Observations at this level are normally used to quantify mycorrhizal associations. |
|
|

|
The higher level of magnification provided by a compound microscope allows mycorrhizal structures within roots and details of spore structure to be seen. |

|
Root fragments that have been cleared and stained are examined to reveal structural details of mycorrhizas. Variations in these structures are sometimes used to identify fungi. |

|
Observations at this scale allow fungi to be identified by the structure of their spores. Spores are normally separated from soil by washing soil through very fine sieves, then concentrated by centrifugation in a dense medium such as a sugar solution. |

|

|

|
| VAM associations form when host roots and compatible fungi are both active in close proximity and soil conditions are favourable. The stages in root colonization by VAM fungi are illustrated below. Associations formed by species of Glomus, Gigaspora, Scutellospora and Acaluospora are shown. Terminology is explained in the glossary. |
| Mycorrhizal associations may be initiated by spore germination as illustrated here. Hyphae may also originate from fragments of roots. In many cases there already is a pre-existing network of hyphae resulting from previous root activity. Hyphae resulting from spore germination have a limited capacity to grow and will die if they do not encounter a susceptible root within a week or so. In Scutellospora species hyphae emerge from a germination shield within the spore. |
|
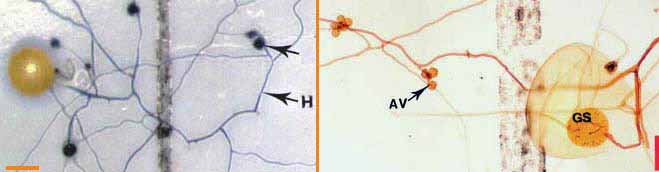
| Germinating hyphae which emerged several days after spores were extracted from dry soil. These spores are Gigaspora (left) and Scutellospora (right) species. |

 These spores were germinated on membrane
filters in contact with soil solution (Brundrett & Juniper 1995).
Hyphae in the picture on the left were stained with Trypan blue. AV =
accessory vesicles (defined below). GS = germination shield. (Bars =
100 um)
These spores were germinated on membrane
filters in contact with soil solution (Brundrett & Juniper 1995).
Hyphae in the picture on the left were stained with Trypan blue. AV =
accessory vesicles (defined below). GS = germination shield. (Bars =
100 um)
|

|
Soil hyphae, also known as extraradical or external hyphae, are
filamentous fungal structures which ramify through the soil. They are
responsible for nutrient acquisition,
propagation of the association, spore formation, etc. VAM fungi produce
different types of soil hyphae including thick "runner" or
"distributive" hyphae as well as thin "absorptive" hyphae (Friese &
Allen 1991). The finer hyphae can produce "branched absorptive
structures" (BAS) where fine hyphae proliferate (Bago et al. 1998).
Hyphae of VAM fungi have been observed to proliferate in soil
microsites where nutrients are concentrated (St John et al. 1983).
Hyphae of Scutellospora and Gigaspora species produce clustered swellings with spines or knobs called auxiliary cells (bodies or vesicles).
The network of hyphae in the soil is only connected to roots by the entry points that initiate mycorrhizal associations, as hyphae do not grow out of living roots. |
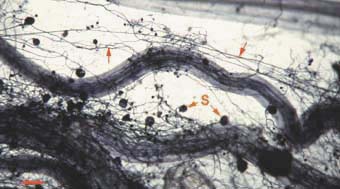
|
Mycorrhizal root system washed
carefully from coarse sand to reveal the intact network with external
hyphae (arrow) with spores (S) produced by Glomus mosseae.
|
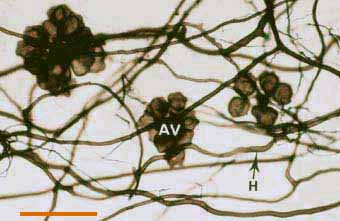
|
 Darkly pigmented soil hyphae (H) of a Scutellospora species with auxiliary cells (AV).
Darkly pigmented soil hyphae (H) of a Scutellospora species with auxiliary cells (AV).
 (Bar = 100 um) |
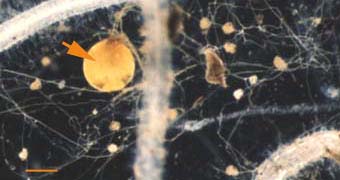
|
Soil hyphae produced by a single germinated spore of Gigaspora
(arrow) used to start a VAM pot culture. This hyphal network has
produced accessory vesicles and is spreading its mycorrhizal
association throughout the root system of a clover plant.
 (Bar = 100 um) |
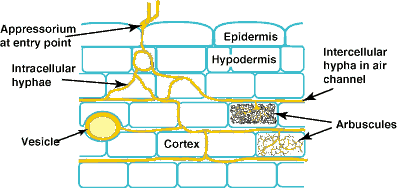
| Mycorrhizal associations start when soil hyphae respond to the presence of a root by growing towards it, establishing contact and growing along its surface. Next, one or more hyphae produce swellings called appressoria between epidermal cells. Root penetration occurs when hyphae from the appressoria penetrate epidermal or cortical cells to enter the root. These hyphae cross the hypodermis (through passage cells if these are present in an exodermis) and start branching in the outer cortex. Relevant root structural features are explained in Section 2. |
 The images in the following sections are of cleared roots which have been stained with Chlorazol black E. Many images are from a study of the time course of VAM formation by Glomus versiforme in leek (Allium porrum) roots (Brundrett et al. 1985).
The images in the following sections are of cleared roots which have been stained with Chlorazol black E. Many images are from a study of the time course of VAM formation by Glomus versiforme in leek (Allium porrum) roots (Brundrett et al. 1985).
|

|
Soil hyphae have
produced 2 appressoria between epidermal cells (arrows). These are seen
here in a surface view of a root with attached hyphae.
|
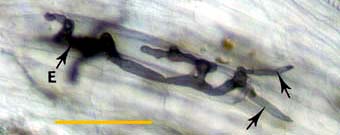
|
Hyphae at an entry point (E) penetrating cortex cells (arrows) approximately 1 day after contact with the root.
 Whole mount focused on outer cortex. (Bar = 100 um) |
|
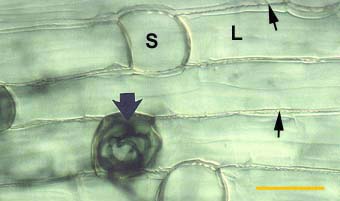
Alternating long (L) and short (S) cells in the dimorphic exodermis of a Smilacina racemosa root. Hyphae of VAM fungi have penetrated unsuberised short cells (arrows).
|
Aseptate hyphae spread along the cortex in both directions from the
entry point to form a colony. Hyphae within root are initially without
cross walls, but these may occur in older roots. Gallaud (1905)
observed that VAM associations in different species formed two
distinctive morphology types, which he named the Arum and Paris
series after host plants. The differences between these two modes of
spread within the root cortex are illustrated below. Both Arum and
Paris type VAM associations are important in ecosystems (Smith &
Smith 1997).
|
|
A colony refers to hyphal growth within a root resulting from the same external hyphae (1 or more connected entry points). These are also called infection units. |

|
Part of a colony of a VAM fungus (Scutellospora sp.) resulting from hyphal growth from an entry point (arrow).
|
| (i) The Paris Type |
| These are associations where hyphae spread primarily by intracellular growth following a convoluted path through cortex cells. The resulting colonies of VAM fungi generally have a coiled appearance (Gallaud 1905, Brundrett & Kendrick 1998), but may have more digitate branching patterns (Widden 1996). Arbuscules may be restricted to a single layer of cells in the inner cortex. |
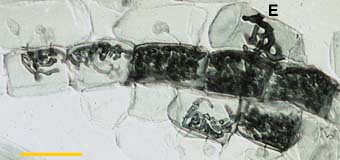 |
Colony of a VAM fungus spreading from the entry point (E) by convoluted hyphae in the inner cortex of an Erythronium americanum root. This hyphal growth pattern is typical of roots without cortical air channels.
 (Bar = 100 um) |
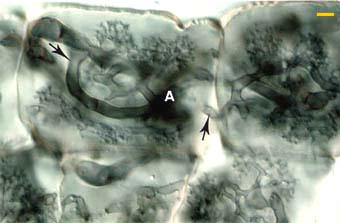 |
Higher magnification view of Arbuscules (A) in the inner cortex of an Erythronium americanum root. Note how arbuscular branched arise from the same hyphae (arrows) which spread the association to new root cells.
View larger images (40 KB)
|
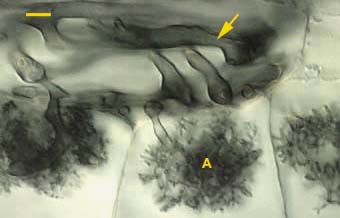 |
Arbuscules (A) and convoluted hyphae (arrow) in the inner cortex of an Asarum canadense root. Arbuscules only form in the innermost cortex cell layer next to the endodermis in this species.
|
| (ii) The Arum Type |
| These are associations where hyphae grow along longitudinal intercellular air channels between the walls of root cells. A relatively rapid parallel spread of intercellular hyphae may occur along these channels. The resulting colonies of VAM fungi have a linear appearance. |
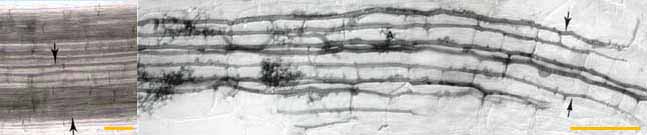 |
| Left: Intercellular
air channels (arrows) in a whole mount of a living leek root, shown for
comparison with mycorrhizal development. These channels run
continuously from the apex to the base of roots. (Bar = 100 um) Right: Longitudinal growth of hyphae of a VAM fungus (Glomus versiforme) along cortex air channels. Note progressive development of arbuscules (A) with increasing distance from the growing tips of hyphae. (Bar = 100 um) |
|
Arbuscules are intricately branched haustoria that formed within a root
cortex cell. They were named by Gallaud (1905), because they look like
little trees. Arbuscules are formed by repeated dichotomous branching
and reductions in hyphal width, starting from an initial trunk hypha
(5-10 um in diameter) and ending in a proliferation of fine branch
hyphae (< 1 um diameter). Arbuscules start to form approximately 2 days after root penetration. They grow inside individual cells of the root cortex, but remain outside their cytoplasm, due to invagination of the plasma membrane. Arbuscules are considered the major site of exchange between the fungus and host. This assumption is based on the large surface area of the arbuscular interface, but has not been confirmed (Smith 1995). Arbuscule formation follows hyphal growth, progressing outwards from the entry point. Arbuscules are short-loved and begin to collapse after a few days, but hyphae and vesicles can remain in roots for months or years. |
 |
Developing arbuscule of Glomus mosseae in a root cell with fine branch hyphae (arrows). The trunk (T) of this arbuscule branched from an intercellular hyphae.
|
 |
Mature arbuscule of Glomus mosseae with numerous fine branch hyphae.
View a larger image of this arbuscule (56 KB)
|
 |
An arbuscule of Glomus versiforme in a root cortex cell with branch hyphae densely packed in the cortex cell of the host.
|
 |
Arbuscule of Gigaspora margarita with an elongated trunk hypha (T) and tufts of fine branch hyphae (arrows). Note how this arbuscule differs from the Glomus arbuscules above.
|
| Vesicles develop to accumulate storage products in many VAM associations. Vesicles are initiated soon after the first arbuscules, but continue to develop when the arbuscules senesce. Vesicles are hyphal swellings in the root cortex that contain lipids and cytoplasm. These may be inter- or intracellular. Vesicles can develop thick walls in older roots and may function as propagules (Biermann & Linderman 1983). Some fungi produce vesicles which are similar in structure to the spores they produced in soil, but in other cases they are different. |
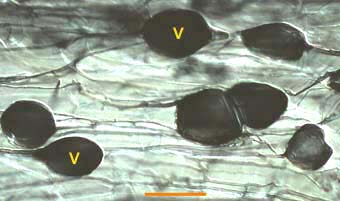 |
Vesicles (V) produced by a Glomus species in a leek root. This root also contains many intercellular hyphae.
|
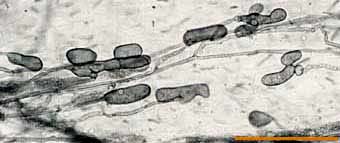 |
Lobed vesicles of an Acaulospora species in a clover root.
|
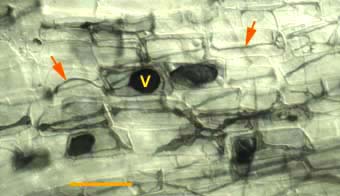 |
Hyphae (arrows) and vesicles (V) of a putative VAM fungus within a rhizome scale of Hydrophyllum virginianum, a nonmycorrhizal plant. This saprophytic activity by VAM fungi can be distinguished by the absence or arbuscules.
 (Bar = 100 um) |
|
It is possible to identify individual Glomalean fungi by recognising
characteristic root morphology patterns in roots (Abbott 1982).
Identification of endophytes within roots is important for culture
quality control, because contaminating fungi can be identified months
before they sporulate (Brundrett et al. 1999). This procedure can also
be used to determine the mycorrhizal inoculum potential of different
fungi by growing trap plants in a soil.
The best way to identify Glomalean fungus colonization in roots is to get to know them by examining single-isolate pot cultures. Mycorrhizal morphology is also influenced by host root structure, so it is best to work with one plant species. It is usually easier to identify fungi in roots with a thick cortex than in species with narrow roots, and species with heavily pigmented roots that are difficult to clear should be avoided if possible. It is generally easy to recognise genera of VAM fungi by their root colonization patterns, but it is also often possible to separate species (especially within Glomus). Morphological features that are important include variations in vesicles (size, shape, wall thickness, wall layers, position and abundance), hyphal branching patterns, the diameter and structure of hyphae (especially near entry points), and the staining intensity of hyphae (dark or faint). Characteristics of genera of Glomalean fungi are listed and illustrated below. |
| Mycorrhizas produced by Glomus species: | |
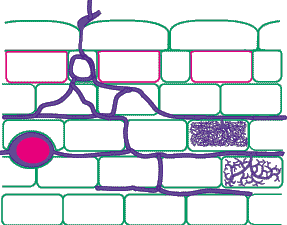 |
|
| Mycorrhizas produced by Scutellospora and Gigaspora species: | |
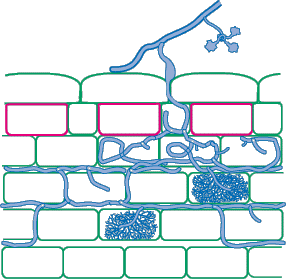 |
|
| Mycorrhizas produced by Acaulospora species: | |
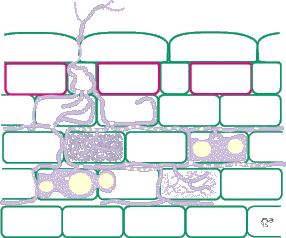 |
|
| Mycorrhizas produced by fine endophytes: | |
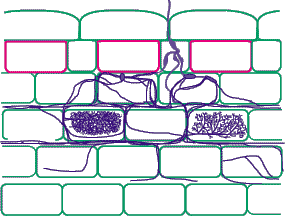 |
|
| Spores form as swellings on one or more subtending hypha in the soil or in roots. These structures contain lipids, cytoplasm and many nuclei. Spores usually develop thick walls with more than one layer and can function as propagules. Spores may be aggregated into groups called sporocarps. Sporocarps may contain specialised hyphae and can be encased in an outer layer (peridium). Spores apparently form when nutrients are remobilised from roots where associations are senescing. They function as storage structures, resting stages and propagules. Spores may form specialised germination structures, or hyphae may emerge through the subtending hyphae or grow directly through the wall. |
 |
The following pictures show some of the fungi isolated into pot cultures started using spores or soil collected from Kakadu National Park in tropical northern Australia (Brundrett et al. 1999). |
| Diversity of spores in a single soil sample | |
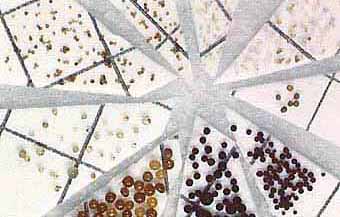 |
 |
 |
Spores can be separated from soil then sorted into categories based on size and colour. The image on the right shows how spores (S) on a piece of filter paper can be used to start a "pot culture" using pasteurised soil in which a host plant will be grown. |
 |
The images below show some of these spores viewed with a compound microscope. Inner wall layers have been revealed by crushing spores and by staining with Melzer's reagent (I2KI) (Bars = 100 um). |
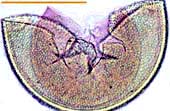
| Spores of Glomus | |||
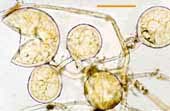 |
Relatively small white spores of a Glomus species. | 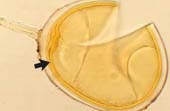 |
Spore of Glomus clarum which has a visible inner wall layer (arrow). |
 |
Sporocarp of Glomus invermaium typical of the dead spores often found in field-collected soil. |  |
Living spores of Glomus invermaium from a pot culture. |
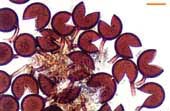
| Spores of Acaulospora | |||
 |
Acaulospora spore with deep pits in the outer wall and inner wall layers stained by Melzer's reagent. |  |
Acaulospora spore with several inner wall layers (arrows). One layer has stained darkly with Melzer's reagent. |
| Spores of Scutellospora | |||
 |
White Scutellospora spores with prominent brown germination shields. |  |
Large black spore with deep pits of Scutellospora reticulata. |
 |  |  |
|
Many species of Glomalean fungi have worldwide distribution patterns
and have apparently adapted to diverse habitats. However, it is known
that soil factors such as pH restrict the distribution of some taxa
(Abbott & Robson 1991) and some of these widespread taxa are now
known to comprise more than one species (Morton 1988). Much of the
functional diversity of Glomalean fungi occurs at the isolate level
rather than species level (Brundrett 1991, Morton & Bentivenga
1994). Consequently, habitat information is as important as knowledge
of the taxonomic identity of fungi, for comparing the results of
experiments, or the selection of isolates for practical use.
Populations of mycorrhizal fungi are thought to have occupied the same soil habitats for millions of years, slowly adapting to changes in site conditions (Trappe & Molina 1986). Changes in populations of Glomalean fungi have been observed when ecosystems are converted to monocultures or severely disturbed, providing indirect evidence for habitat preferences by these fungi (Abbott & Robson 1991, Brundrett 1991). There is also experimental evidence that the performance (measured as increased host plant growth) of fungal isolates is related to environmental factors (Brundrett 1991, Abbott et al. 1992). However, we do not enough about the functional diversity of Glomalean fungi to understand the importance of changes in their diversity. The classification of the Glomales is largely based on the structure of their soil-borne resting spores, but more recently the careful study of developmental processes and biochemical properties have provided valuable information (Walker 1992, Morton 1993). Accurate identification of Glomalean fungi often requires them to be isolated in cultures with host plants, to observe developmental stages and avoid the loss of diagnostic features which occurs in field-collected material. |
 |  |  |
Summary
Classification scheme for Glomalean taxa (Morton & Benny 1990)
Only general information on the classification of Glomalean fungi is provided here. The following sites should be consulted for more information about these fungi: |
 |
 |
 |
| Families and genera of host plants for this association include a large proportion of the vascular plants, so are too numerous to list here. A list of Australian plants which have been shown to have VAM is provided. |
 |
 |
 |
This glossary defines important terminology used to describe vesicular-arbuscular mycorrhizas (VAM).
|

Home Page |
 CSIRO Forestry and Forest Products |
 Australian Centre for International Agricultural Research |
| This page was last revised October 14 1999 | ||
 Start Here |
 Intro- duction |
 Roots |
 Top of this page |
 ECM |
 Roles |
 Australian Plants |
 Eucalypt Associates |
 Methods |
 Refer- ences |